I often see all types of questions on the use of oxygen absorbers and Mylar bags, so I thought it was time to write a blog addressing some of them.
I use a simple rule when I pack food in Mylar bags and buckets. If it is a number 10 metal can or a one-gallon Mylar bag, I use a 300 cc oxygen absorber. In all the packaging I write about, I will use the 300 cc O2 absorber. The reason is I get them from an LDS cannery that is convenient for me, and that is the only size they carry. My rule is one absorber per gallon, regardless of what the product is. In other words a five-gallon bucket with a large Mylar bag filling it would take five absorbers.
Now oxygen absorber packets should remove oxygen from airtight containers to below 0.01%, according to Food Industry standards, when used as directed. The primary ingredients in the absorbers are salt and iron. Upon exposure to air, the iron in the packet immediately begins absorbing oxygen and breaks down into harmless iron oxide which is safely contained in the packet and does not contaminate the food.
For those who want to be more precise than me, I have included information from Sorbent Systems a large distributor of food preserving products.
The following table indicates how many cc’s of oxygen are contained in the more common sizes of food storage containers.
| Container Type | Volume in Empty Container |
| #10 can | 3,980 cc |
| 5 gallon plastic pail | 18,942 cc |
| 6 gallon plastic pail | 22,730.4 cc |
Oxygen absorbers are rated by their capacity to absorb oxygen as measured in cubic centimeters (cc). There are two key elements to keep in mind when determining what size of oxygen absorber to use:
Headspace
As in the table above, a #10 can has a volume of 3,980 cc. If you fill this can to 90% of its volume, you will have 398 cubic centimeters of headspace.
Because air is 21.0% oxygen, this headspace will contain 81.6 cubic centimeters of oxygen (0.21 x 398 = 81.6).
Voidspace
If a five-gallon bucket were filled with children’s marbles, the spaces in between the marbles (void spaces and head space) would represent 38% of the volume of the five-gallon pail ( 0.38 x 18,942 cc = 7,197.96 cc residual air volume ). Of this number, 21.0% is oxygen.
(x) cc Headspace + (x) cc Voidspace = Residual Air Volume
In order to determine how much oxygen absorbing capacity you require, we must determine the residual air volume. Here is a fairly simple way of determining this quantity, using weight and volume measurements.
1. Determine the volume of your container. Use the table above for common container types.
2. Weigh the food product and convert this weight to grams. To be completely accurate, make sure you subtract the weight of the container to get the net weight of the food.
3. Subtract the cubic centimeter volume found in Step 1 above from the gram weight found in Step 2 above to determine the headspace and void space, or residual air volume. (This will be a measurement in cubic centimeters)
4. Finally, as there is approximately 21.0% oxygen in air, multiply the residual air volume found in Step 3 above by .21 to get the cubic centimeter volume of oxygen in your product container.
Example:
We want to know what size oxygen absorber to use for a 5-gallon bucket of rice.
1. We determine from the table above that there are 18,942 cubic centimeters in a 5-gallon plastic bucket.
2. The rice weighs 35 pounds which converts to 15,876 grams.
3. 18,942 (cc) container volume – 15,876 (g) rice = 3,066 cc residual air volume.
4. 3,066 (cc) residual air volume x .21 (oxygen fraction in air) = 628.53 cc oxygen volume
5. Since the size of oxygen absorbers are rated and named according to the amount of oxygen they absorb, we know that a 750 cc oxygen absorber will be sufficient for this bucket of rice
If your container is a regular shape such as in the diagram below, use the formula which follows:
NOTE: one milliliter (ml) = one cubic centimeter (cc)
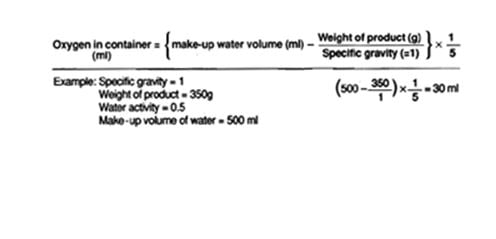
If your container is an irregular shape, such as a flexible mylar® bag, the oxygen volume can be determined by doing the following:
For purpose of this calculation, we are going to assume that the specific gravity of the product has a value of 1.
1. Fill a vessel with water. Place your product container (containing the food) into the vessel and let the water overflow.
2. Remove your container from the vessel.
3. Measure how much water it takes to fill the vessel back up with water. The water is measured in milliliters (ml). There are 29.57 ml in one fluid ounce.
4. Measure the weight of the product container in grams (g). There are 28.35 grams in an ounce.
The formula for this is as follows:
NOTE: one milliliter (ml) = one cubic centimeter (cc)
In the example above, the oxygen in the container is 30 ml. Therefore, an oxygen absorber will have to be at least 30 cc. Our 50 cc oxygen absorber will be more than sufficient.
Specific Gravity
Relative density (measure of the density of a substance compared with that of a standard) when the standard substance is pure water.
| MEASUREMENT CONVERSION TABLE | ||
| To Convert: | To: | Multiply by: |
| inches | centimeters | 2.54 |
| square inches | square centimeters | 6.54 |
| cubic inches | cubic centimeters | 16.387 |
| fluid ounces | milliliters | 29.57 |
| ounces (weight) | grams | 28.35 |
Top of Form
How do I know when an oxygen absorber is used up or no good?
This is one of the most frequently asked questions we get. The easiest way to tell if an oxygen absorber is good is to pinch the packet. If it feels ‘soft’ or powdery, the iron oxide powder is still in its original state and it is good. If it feels ‘hard’ or like a solid wafer in the packet, it is completely spent and should be replaced.
How tight should the absorbers suck the bag down?
Many people seem to think that if the bag does not suck down tight it has failed to work. Due to the varying air space between the contents, you cannot use this as a reliable indicator. The absorber only removes roughly 1/5 of the air from the void. How tight the bags suck down depends on a couple of factors, one how much headspace did you leave in the container and two how much larger is the void between the grains. In fact, if the bags suck down too tight it can cause pinhole leaks.





I read the other day LDS Cannery had been shut down.
That is incorrect. You will still be abled to buy products for the same price, but they will already be canned. This a cost cutting measure.
Howard
I have this darn science background and I get sensitive to unit analysis.
In your example subtracting grams from cc’s does not give cc’s. I think you need some sort of measure of voidspace per gram — and I suspect that kidney beans have a different result than does rice.
I agree with Brian. Unless you assume one gram weight per CC of food volume, then the formulas are highly suspect. That might be a reasonable assumption. Dunno.
I’ve found that generalizations are fine with food storage and oxy absorbers, these formulas notwithstanding. We use 300cc absorbers with most of our packaged food.
Can you use too many oxy absorbers… will it cause harm ? I just have big ones and have smaller buckets?
Do I need to shake/rotate the contents of a 10 gallon bucket or will the oxygen eventually be absorbed without disturbing the bucket?